Apply
The application process for summer 2024 is now closed. Award notifications will begin in March. Application materials are still posted for reference only.
Application Instructions
 Fellowship recipient Alfred Chang
Fellowship recipient Alfred Chang
1) Review the Frequently Asked Questions.
2) Review the summer 2024 NURF project descriptions (below) and select one or more project(s) of interest.
3) Complete the APPLICATION, and save the file with your last name and your first name as the first words in the file name. (Ex: Smith-Barb 2024 NURF Application)
4) By 8:00am Eastern on February 7, email your completed application and current resumé (saved with the same name convention -- Smith-Barb 2024 NURF resumé) to the project’s faculty mentor(s) for consideration, and cc: ndnano@nd.edu. NDnano faculty will follow-up with selected applicants directly. Award notifications will begin in early March.
Students are welcome to apply for more than one project. However, please list and prioritize on your application(s) all the projects for which you are applying. Please include project title and faculty/mentor first/last name.
VIDEO: OVERVIEW OF NDNANO UNDERGRADUATE RESEARCH FELLOWSHIPS
VIDEOS: WHY CHOOSE NOTRE DAME FOR YOUR SUMMER RESEARCH?
Still have questions?
Feel free to contact Heidi Deethardt.
Please Note
For purposes of the NURF program, undergraduates are students who will not yet have completed their undergraduate studies at the start of their summer fellowship.
The University allows students to work a maximum of 40 hours per week during the summer (all campus jobs combined). This means that students cannot participate in the NURF program on a full-time basis and at the same time hold any other paid, on-campus, summer position. (Fellowships of less than 40 hours per week (i.e., to also work for Residential Life) would be considered on a case-by-case basis.)
In addition, fellowship recipients who attend Notre Dame and have 2023-2024 on-campus, academic-year jobs may have restrictions on their NURF start date; contact Heidi Deethardt for more details.
Summer 2024 NURF Project Descriptions
Project title: Polymer crystallization for automated material synthesis
Faculty mentors:
Professor Gabriel Burks • Chemical and Biomolecular Engineering • 205B McCourtney Hall • 574-631-2534 • gburks2@nd.edu (Send applications to Professor Burks)
Professor Yamil Colon • Chemical and Biomolecular Engineering • 368 Nieuwland Hall • 574-631-8246 • ycolon@nd.edu
In this summer project, our primary objective is to contribute to the advancement of sustainable material development by designing polymer crystals specifically tailored for microfluidics-based imaging. The urgent need for environmentally conscious material synthesis methods necessitates a deeper understanding of the structural nuances of polymers. By leveraging microfluidics devices as controlled environments, we aim to create a platform for imaging polymer crystals with precision. The acquired data will enhance our understanding of polymer structures and simultaneously be used to develop training datasets for machine learning-driven automated material fabrication workflows. This project aligns with the broader goal of promoting closed-loop materials synthesis, aiming to establish a sustainable and circular approach to material design, usage, and recovery. By employing microfluidics and advanced imaging techniques, we aspire to improve the efficiency of material fabrication processes and to contribute to the broader vision of minimizing the harmful environmental footprint of synthetic polymer and synthetic polymer-derived devices.
Under the umbrella of this project, the student will share time:
- fabricating and imaging polymer crystals;
- organizing and cleaning image data; and
- training machine learning models with image data
This project sits at the intersection of chemistry, physics, engineering, and computer science. Applicants should be open to both experimental and computational workflows, and have some working understanding of programming.
Project: Circuit and system design for collaborative machine learning
Faculty mentor:
Professor Ningyuan Cao • Electrical Engineering • 226A Cushing Hall • 574-631-6618 • ncao@nd.edu
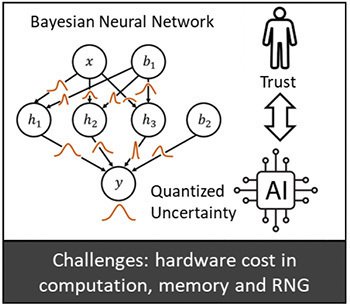
In today's era of rapid technological advancement, machine learning has found its application across a multitude of fields, including but not limited to healthcare, finance, automotive, entertainment, and agriculture. However, a major challenge with many machine learning models, especially deep neural networks, is their "black-box" nature. This opaqueness often poses hurdles in establishing trust between AI systems and human users. One key aspect of this challenge is the inability of these models to effectively communicate the confidence or uncertainty associated with their predictions. In contrast, statistical machine learning approaches, like Bayesian inference and Bayesian neural networks (BNN), offer a unique advantage. Rather than focusing solely on finding an optimal model with fixed weights, they aim to learn the distribution of model parameters. This intrinsic capability allows Bayesian approaches to quantify and convey the uncertainty related to their learning processes, thereby fostering enhanced trust and collaboration between AI and humans. While powerful, such statistical machine learning approaches are notably resource-intensive. The requirement for massive random number generation, coupled with extensive computation to assess inference confidence, can be prohibitive. This computational burden makes them challenging to deploy on resource-constrained edge devices, which are often the primary platforms directly interfacing with human users.
This project aims to bridge this gap by offering participants a comprehensive training regimen, focused on the theoretical and practical aspects of analyzing and designing Bayesian Neural Networks on constrained devices. The training components include:
- Algorithm and Simulation: The student will dive deep into the theoretical foundations of Bayesian theory and its application in machine learning with hands-on model building experience from scratch.
- Hardware Cost Analysis: The training will provide insights into the granular breakdown of BNN computations, covering aspects like random number generation, distribution management, memory access, and more. This will enable the student to understand the challenges and bottlenecks associated with deploying BNNs on hardware.
- Circuit Analysis and Design: A pivotal component of this project is the design and evaluation of innovative circuits aimed at accelerating Bayesian approaches. The student will be guided through the process of circuit analysis, design considerations, and prototyping, ensuring that their solutions are apt for resource-constrained environments.
- Full-stack Capabilities: To ensure holistic learning, the student will also receive training on Python programming, architecture emulation, and various simulation tools. This full-stack approach will empower the student with the versatility needed to tackle real-world challenges.
Applicants of diverse backgrounds are welcome, including computer science, computer architecture, machine learning, circuit design, and electronic devices.
Project: Elucidate electronic structure and charge transfer in hybrid bronzes
Faculty mentors:
Professor Hsing-Ta Chen • Chemistry and Biochemistry • 373 Nieuwland Hall • 574-631-1935 • hchen25@nd.edu (Send applications to Professor Chen)
Professor Adam Jaffe • Chemistry and Biochemistry • 375 Stepan Hall • 574-631-2696 • ajaffe@nd.edu
This project aims to elucidate the electronic structure and charge transfer mechanisms of a variety of hybrid bronzes using first-principle atomic simulations. Hybrid bronzes are novel materials that place molecular layers with tunable functional groups in close contact with inorganic layers that possess high electron mobility, which allows for control over electronic conductivity and inter-layer charge transport. Gaining fundamental insight into the relationship between the electronic properties and charge transfer in hybrid bronzes is important for future applications in nanotechnologies.
In this project, the student will advance recent research in characterizing hybrid bronzes developed at Notre Dame using first-principle computation packages. The student will be responsible for (1) performing geometry optimization of the crystal structure using various levels of electronic structure theory; (2) calculating the band structure and projected density of states of individual atoms/elements; and (3) analyzing the overlap of molecular orbitals with the inorganic layer to construct a tight-binding model for electron transfer mechanism. Highly self-motivated students with a strong background in physical chemistry and basic knowledge of quantum chemistry are welcome to apply. Coding experience, particularly in Python, is preferred though not required.
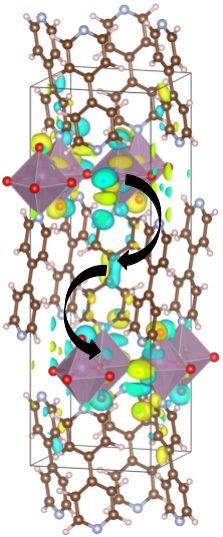
Figure: The wavefunction distribution of Mo-based hybrid bronze. The inorganic layers contain Mo atoms coordinated by red O atoms. The molecular layer is bipyridine directly connected to the Mo atom. The arrow shows inter-layer charge transfer pathways.
Project: Nanoparticle mediated reprogramming of cells to fight cancer
Faculty mentors:
Professor Meenal Datta • Aerospace and Mechanical Engineering, Bioengineering Graduate Program • 145 Multidisciplinary Research Building • 574-631-5735 • mdatta@nd.edu (Send applications to Professor Datta)
Professor Ryan K. Roeder • Aerospace and Mechanical Engineering, Bioengineering Graduate Program • 148 Multidisciplinary Research Building • 574-631-7003 • rroeder@nd.edu
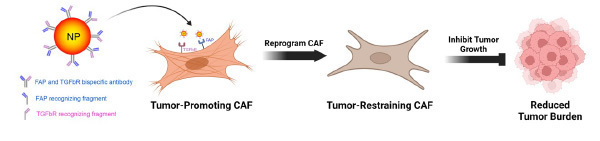
(that bind to FAP and TGFbR receptors on CAF cell membranes) and loaded with
chemokines to target and convert tumor-promoting CAFs into tumor-restraining CAFs,
thus reducing tumor growth and enhancing sensitivity to therapy.
In difficult-to-treat cancers, normal host cells are co-opted to facilitate cancer growth and spread. Fibroblasts normally provide support and structure to our tissues, but once re-programmed in the presence of cancer, carcinoma-associated fibroblasts (CAFs) fuel tumor progression, metastasis, immunosuppression, and treatment resistance. However, fibroblasts are highly plastic, meaning they are able to tune their behavior in response to specific biological, chemical, and mechanical cues from their surrounding environment. Therefore, nanoparticles can be applied to reprogram CAFs to fight against – rather than support – tumors (Figure 1).
This multidisciplinary project melds tumor model and intravital imaging (e.g., live in an animal) expertise in the Datta Lab with nanoparticle design and imaging expertise from the Roeder Lab. The undergraduate student will be mentored by a graduate student from each lab and will be responsible for nanoparticle synthesis and optimization, fibroblast cell culture assay development and analysis, and early stage in vivo administration and visualization of the nanoparticles. The undergraduate student should be pursuing a STEM major and should have completed introductory biology and chemistry with associated laboratory components by summer 2024. Prior wet-lab experience (e.g., cell culture, molecular assays, nanoparticle synthesis, animal handling, etc.) by candidates is desirable but not required.
Project: Pursuing superconductivity in doped transition metal dichalcogenides
Mentors:
Professor László Forró • Stavropoulos Center for Complex Quantum Matter; Physics and Astronomy • 433 Nieuwland Science Hall • 574-631-7012 • lforro@nd.edu
Dr. Bence Márkus • Stavropoulos Center for Complex Quantum Matter; Physics and Astronomy • 442 Nieuwland Science Hall • 574-631-4164 • bmarkus@nd.edu (Send applications to Dr. Markus and Anna Nyary)
Anna Nyáry • Stavropoulos Center for Complex Quantum Matter; Physics and Astronomy • 442 Nieuwland Science Hall • anyary@nd.edu
This project aims to prepare and investigate novel superconductors, such as n- and p-doped transition metal dichalcogenides (TMDCs). Superconductivity represents a compelling physical phenomenon that has inspired both theoretical advances and resulted in numerous applications. The quest for new superconductors, especially in materials with layered structures, continues. The purpose of this study is to prepare new materials that advance the field of superconductivity. The materials are going to be characterized with SQUID/VSM standard magnetometry methods and/or with transport methods. The results of this study help will the student to deepen their knowledge (both theoretical and experimental techniques) and give new insights into the field. Numerous industries are interested in novel superconductors that can outperform current ones either in transitional temperature, critical field, or in price-effectiveness. The student will work closely with ND postdocs and graduate students on sample synthesis, investigation, and data analysis.
Applicants with experimental background, basic knowledge of superconductivity, basic knowledge of handling hazardous materials, and knowledge of magnetometry methods (SQUID/VSM) or transport techniques (DC/µW) are a plus.
Project: Tailoring the electronic/magnetic properties of MXenes
Mentors:
Professor László Forró • Stavropoulos Center for Complex Quantum Matter; Physics and Astronomy • 433 Nieuwland Science Hall • 574-631-7012 • lforro@nd.edu
Dr. Bence Márkus • Stavropoulos Center for Complex Quantum Matter; Physics and Astronomy • 442 Nieuwland Science Hall • 574-631-4164 • bmarkus@nd.edu (Send applications to Dr. Markus and Dr. Beke)
Dr. Dávid Beke • Stavropoulos Center for Complex Quantum Matter; Chemistry • 442 Nieuwland Science Hall • 574-631-7361 • dbeke@nd.edu
The need for more and more computational power, data storage, and the storage of energy is one of the crucial problems mankind faces nowadays. The target of this project is to discover and tune/tweak new materials that might find applications in the field of spintronics and energy storage. The student will prepare and investigate MXenes, a new family of low-dimensional materials, and try to modify their properties with various dopants. The obtained materials are going to be characterized with XRD, electron spin resonance (ESR), and Raman spectroscopy. During the work, the student will learn about new synthesis techniques, and the operation of the above-mentioned instrumentation, and learn about the arising physical phenomena. The student will work closely with ND postdocs and graduate students on sample synthesis, investigation, and data analysis.
Applicants with experimental background, basic knowledge of handling glovebox and vacuum systems, and knowledge of magnetic resonance spectroscopic (ESR) methods and Raman spectroscopy are a plus.
Project: Development of perovskite-based solar cells
Mentors:
Professor László Forró • Stavropoulos Center for Complex Quantum Matter; Physics and Astronomy • 433 Nieuwland Science Hall • 574-631-7012 • lforro@nd.edu
Dr. Dávid Beke • Stavropoulos Center for Complex Quantum Matter; Chemistry • 442 Nieuwland Science Hall • 574-631-7361 • dbeke@nd.edu (Send applications to Dr. Beke)
With the growing awareness among individuals and the strict permissible discharge levels set by regulatory authorities in various countries, there is a need for technological advancement to achieve circular economy targets in heavy metal-containing technologies. The latest technologies that use lead in optoelectronic research, especially in photovoltaics, are based on organic-inorganic lead halide perovskite semiconductors. The aim of the summer research program is to contribute to the development of state-of-the-art perovskite-based systems by utilizing materials chemistry and condensed matter physics to address the current challenges that perovskite-based systems face.
The student will have the opportunity to gain hands-on experience in synthesizing materials using a variety of techniques. They will also be responsible for characterizing the synthesized materials utilizing advanced tools like PPMS, Raman microscopy, electron spin resonance spectroscopy, and electrical measurements. In addition, the student will be trained to analyze and interpret data obtained from these instruments and present their findings in a clear and concise manner.
Project: Biocompatible membrane coated nanoelectrode for sweat analyte measurements
Faculty mentors:
Professor Kaiyu Fu • Chemistry and Biochemistry • 140D McCourtney Hall • 574-631-1853 • kfu@nd.edu (Send applications to Professor Fu)
Professor William Phillip • Chemical and Biomolecular Engineering • 205F McCourtney Hall • 574-631-2708 • wphillip@nd.edu
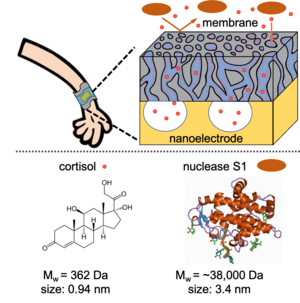
In recent years, wearable devices, such as the Apple Watch and Fitbit, have paved the way toward real-time monitoring of physiological signals (e.g., temperature, electrocardiogram (ECG), blood O2 levels) in a personalized and continuous manner. As wearable device designs advance, a critical need exists for innovative solutions that enable the measurement of biomolecules and chemicals excreted from sweat over extended periods of time. For instance, the quantification of stress hormones, such as cortisol, is relevant to controlling stress disorders and monitoring the mental health of human beings. Sensor fouling (i.e., the non-specific deposition of material on the sensing interface) is one of the major technical barriers to achieving the long-term use of these devices for monitoring cortisol levels in sweat. Resolving this challenge necessitates the development of an interface that allows for efficient signal transduction while addressing broad challenges relevant to device fouling. So far, the dominant strategy for device protection is to deposit a barrier layer between the biofluids and the device. However, it is difficult to integrate other functional elements that promote the selective transport of sweat analytes to the biosensing surface within the protective antifouling layer.
In this project, we propose to meet this demand by designing a nanostructured biocompatible membrane with robust formation mechanisms rooted in self-assembly. The membranes that result from this scalable manufacturing process possess tunable pore wall chemistries that can be tailored post-synthetically to afford both antifouling characteristics that prevent non-specific protein adsorption as well as rapid and selective analyte transport mechanisms.
The goal of the proposed research is to develop the fundamental scientific knowledge that informs the design and fabrication of protective gatekeeping membranes tailored to facilitate the transport of sweat analytes (e.g., cortisol) to sensing electrodes. In contributing to this ambitious, potentially transformative project, the student researcher will contribute to three broadly organized objectives:
- Identify molecular design strategies for membranes tailor-made to promote the efficient transport of cortisol by elucidating how the nanoscale structure and chemistry of the membranes impact the experimentally observed transport properties;
- Validate the biosensing performance of cortisol detection using nanostructured electrodes integrated with the membranes developed in objective 1;
- Test the detection performance of the sweat analyte in the presence of common fouling interferences and evaluate the signal stability over a period of time.
Self-motivated, independent student researchers will have the ability to focus their research efforts on the aspect of the project that appeals to their skills and interests. A student trained in chemistry, chemical engineering, mechanical engineering, or electrical engineering with a strong interest in the areas of nanotechnology, analytical chemistry, and polymeric materials are well-suited to undertake this research project.
Project: Development of electrochemical biosensors for drug detection and monitoring in cell culture environment
Faculty mentors:
Professor Kaiyu Fu • Chemistry and Biochemistry • 140D McCourtney Hall • 574-631-1853 • kfu@nd.edu (Send applications to Professor Fu)
Professor Yichun Wang • Chemical and Biomolecular Engineering • 240C McCourtney Hall • 574-631-2617 • ywang65@nd.edu
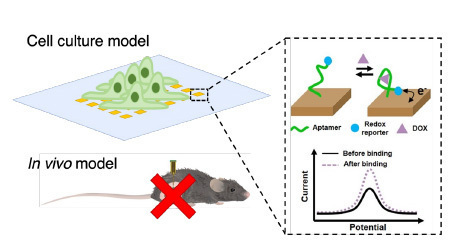
drug detection and monitoring.
Therapeutic drug monitoring usually has a range between effective therapeutic concentration and minimum toxic concentration, which is a narrow therapeutic window that varies among patients of different ages, health conditions, etc. Instead of infrequent drug measurements from static blood samples, real-time drug detection is emerging as a powerful and effective method to reflect the pharmacokinetics in a therapeutic period that lasts from hours to days. In the past decade, electrochemical aptamer-based (EAB) sensors have served as a versatile solution to track and monitor small molecules (metabolites, chemotherapeutics, antibiotics) with good sensitivity, excellent specificity and highly reversible signal generation. We recently reported the first of its kind, a real-time drug monitor that measures pharmacokinetics in tumor tissue from living animals (Science Advances, 2022, 8, abk2901). However, this drug monitoring requires an animal model to evaluate drug performance, which slows down the screening process of potential candidates.
In contrast, 3D cell cultures enable a high-throughput drug screening. They cover a wide range from cell aggregates (spheroids), tissue-like cell models (organoids) to a more complex organ-on-a-chip system that mimics the in vivo environment for drug delivery and measurement (Trends in Pharmacological Sciences, 2022, 43, 569; Frontiers in Chemistry 2023, 11).
In this project, we propose to advance the current technology by integrating the EAB sensor with a 3D cell culture platform for real-time drug delivery and measurement in tumor spheroid. To accomplish this, the student researcher will be involved in three broadly defined objectives:
- Immobilize aptamers on microelectrode arrays and validate drug sensing performance from multiple channels at different electrode sites.
- Grow cancerous cellular spheroids on the culture dish using hanging drop methods.
- Validate cell growth on the electrode surface and test drug response if time permits.
Project: Characterization and optimization of hydrogel coated biosensors with long-term fouling resistance
Faculty mentors:
Professor Kaiyu Fu • Chemistry and Biochemistry • 140D McCourtney Hall • 574-631-1853 • kfu@nd.edu (Send applications to Professor Fu)
Professor Matthew Webber • Chemical and Biomolecular Engineering • 105F McCourtney Hall • 574-631-4246 • mwebber@nd.edu
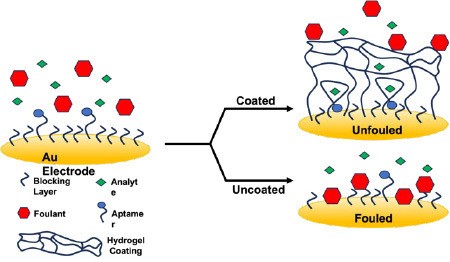
anti-fouling for the detection of analytes.
A long-standing problem in the implementation of biomedical devices for chronic disease monitoring is the biofouling problem when exposed to the biological fluidic environment. Hydrogels have attracted attention for their good biocompatibility, tunable anti-fouling capabilities and desirable mechanical properties. Their intrinsic porous structure can absorb large amounts of water when cross-linked to form a hydrophilic network, which is not only an ideal coating for surface protection of biosensors, but also serves as an active element to selectively transport molecules of interest for detection.
The preparation of hydrogel coatings by surface polymerization offers several advantages over physical adhesion. Strong covalent attachment to the surface prevents delamination of the coating under a variety of conditions. In addition, crosslinking after polymerization would allow for easily controllable crosslink density and customizable crosslinker structure. While hydrogels have been used in various aspects for different biosensing methods, there has been little research on their use as anti-fouling coatings in electrochemical aptamer-based biosensors (EABs). EABs utilize aptamers modified with a redox probe to provide biosensing with low detection limits and high selectivity. Aptamers, which are short-chain oligonucleotides that selectively bind specific targets and undergo conformational changes upon binding, have been used in a variety of biosensing applications.
To accomplish the above research goal, the proposed project is to develop and characterize hydrogel coatings for their efficacy as permeable anti-fouling coatings. The student researcher will be involved in three broadly defined objectives:
- Perform post-polymerization crosslinking and hydrogel formation using azide-functionalized oligoethylene glycol derivatives and copper-catalyzed azide-alkyne click chemistry.
- Characterize functionalized surfaces using X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and ellipsometry.
- Vary the crosslinking density and chemical structure of proposed crosslinkers to investigate how crosslinking affects both antifouling performance and small molecule analyte permeability in gold-electrode-based EABs.
Project: Characterization of plasma jets in open and enclosed environments for nanomaterial processing
Faculty mentor:
Professor David Go • Aerospace and Mechanical Engineering, Chemical and Biomolecular Engineering • 115 Main Building • 574-631-8394 • dgo@nd.edu
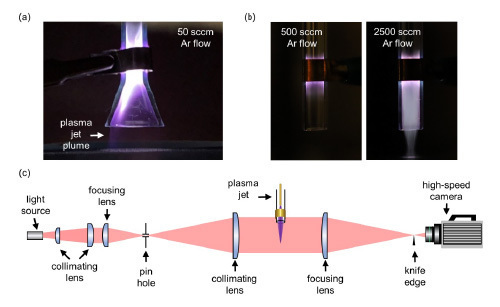
an enclosed stainless-steel chamber using a 50 sccm Ar gas flow,
image adapted from Lee et al., ACS Appl. Mater. Interfaces 13, 47, 56242–56253 (2021).
Copyright © 2021 American Chemical Society. (b) Plasma jet operating in
open space produced constricted discharges around the interelectrode
region using an Ar gas flow of 500 sccm and extended out of the tube with a
substantially higher flow rate of 2500 sccm. (c) A schematic of Schlieren imaging
setup that is planning to be implemented in this work to visualize the gas mixing.
The technical objective of this National Science Foundation-sponsored project is to investigate the possible underlying mechanisms that result in the distinct behaviors of an atmospheric pressure plasma jet when operating within an enclosed chamber compared to operating in open air.
Plasma jets are repetitive ionization waves that are commonly ignited in a noble gas (e.g., He and Ar) flowing through a dielectric tube, expanding into open space with a confined shape. This nature of extending the discharge region outside of the interelectrode gap enables interactions with remote subjects, allowing for applications in various scenarios, including medical therapies, water purification, and most recently, room-temperature sintering of thin films containing nanomaterials.
Our previous research with plasma jets observed an unexpected phenomenon: stable plasma jets with a uniform plume can be easily generated and sustained inside an enclosed reactor using a low noble gas flow rate of ≤ 100 sccm. In contrast, in open air, the discharges are constricted around the interelectrode region unless a substantial volume of noble gas is flowed through. A preliminary hypothesis attributes this observed variation to the different mixing of the jet and background gases, yet the correlation with the propagation of ionization waves remains elusive. This project would primarily be conducted experimentally in the hope of providing physical insight that supports or refutes the hypothesis. Fluid and plasma simulations may also be employed to complement experimental results to verify some key findings.
The role of the student is to assist in the development and characterization of plasma jets operating in open air and within an enclosed chamber. They will collaborate closely with postdoctoral researchers who have expertise in plasma science to conduct experiments such as phase-locked imaging and Schlieren imaging, engage in theoretical analysis, and possibly perform computational modeling using COMSOL Multiphysics, ANSYS Fluent, etc.
Applicants in any science and engineering discipline are welcome to apply, although those with a background in mechanical engineering and electrical engineering are preferred. Students interested in continuing the research throughout the school year will be given priority.
Project: Circular beam semiconductor laser optimization and reliability
Faculty mentors:
Professor Douglas Hall • Electrical Engineering • 260 Fitzpatrick Hall • 574-631-8631 • dhall@nd.edu (Send applications to Professor Hall)
Professor Masaru Kuno • Chemistry and Biochemistry, Physics and Astronomy • 110 Stepan Hall • 574-631-0494 • mkuno@nd.edu
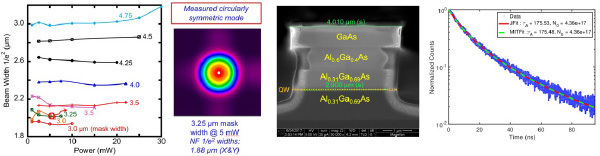
showing stable, single-mode operation; (b) measured circular laser output;
(c) oxide-confined deep-etched AlGaAs/GaAs ridge waveguide laser SEM cross section; (d) TRPL data.
We have developed a deep-etched ridge waveguide edge-emitting laser that uses a special oxidation process to smooth and passivate the sidewall, overcoming high scattering loss and sidewall recombination issues which have plagued their prior development. The invention utilizes an oxygen-enhanced wet thermal oxidation process pioneered by Professor Hall and enables unique high-index-contrast strong-optical confinement waveguides which, due to a demonstrated oxidation smoothing mechanism, benefit from sidewall roughness smoothing to reduce losses. This innovation enabled the recent demonstration of a novel circular mode EEL without the need for complex integrated mode converters or external optics, dramatically reducing the cost of high beam quality lasers and expanding their possible market size to many more applications and enhancing achievable performance of existing ones. Longer range, an effective, viable deep-etched process for active devices also promises to enable advances in future GaAs photonic integrated circuits that require the very low bend loss we have previously demonstrated.
We are currently actively evaluating both the quality of the oxide-passivated sidewall interface and the reliability of laser fabricated by this process. As time resolved photoluminescence (TRPL) is the best method to directly assess sidewall recombination in isolation from the scattering loss reduction effect, we are collaborating with Professor Kuno to perform experiments using their versatile pulsed laser micro-photoluminescence (μPL) system to obtain the surface recombination velocity vs. a measure of interface quality. This powerful technique will yield new insight into fundamental interface properties and enable process optimization to improve laser performance and reliability. Concurrently, we are fabricating lasers in the Notre Dame Nanofabrication Facility, collaborating with industry partners assisting with laser mirror passivation coatings and heatsinking, and performing burn-in and life testing in our custom built 10 channel laser reliability testbed.
A student working on this project will assist an electrical engineering graduate student with TRPL measurements, diode laser characterization, and laser reliability testing (including further development of the testbed). Applicants should be majoring in electrical engineering, physics, chemistry or nanotechnology/nanoscience, preferably with coursework in semiconductor and/or optics. Desirable skills include programming for working with a Teensy 4.1 microcontroller used for data acquisition; Python for testbed automation, and MATLAB for data processing/analysis. Experience with semiconductor device fabrication is useful but not required.
Publication and video presentation from 2021 International Semiconductor Laser Conference (ISLC):
https://ieeexplore.ieee.org/document/9615746
https://notredame.app.box.com/v/Li-etal-ISLC2021
Project: Engineering biomimetic materials to control stem cell morphogenesis
Faculty mentor:
Professor Donny Hanjaya-Putra • Aerospace and Mechanical Engineering, Bioengineering Graduate Program • 141 Multidisciplinary Research Building • 574-631-2291 • dputra1@nd.edu
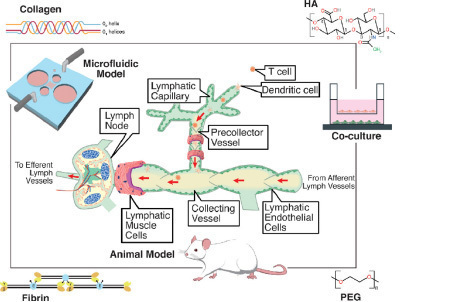
Blood and lymphatic vasculatures are two important components of the tumor microenvironments. Blood vessels supply nutrients important for tumor growth and serve as a conduit for hematogenous tumor spread, while the lymphatic vessels are used by the cancer cells to interact with the immune system as well as for lymphatic tumor metastasis. Consequently, the growth of blood and lymphatic vasculatures surrounding the tumor have been associated with tumor metastases and poor patient prognosis. The objective of this project is to understand what governs the formation of blood and lymphatic vessels from stem cells, how these processes are affected by the tumor microenvironment, and how we can use these insights to develop novel therapies. The student will synthesize and characterize biomaterials for in vitro evaluation using stem cell. The student is expected to maintain stem cell culture, and study cell-materials interaction using microscopy and molecular biology techniques. Students with a background in mechanical engineering, chemical/bioengineering, material science, or biochemistry are encouraged to apply. Prior lab experience is preferred.
Publications:
- Laura Alderfer, Elizabeth Russo, Adriana Archilla, Brian Coe, Donny Hanjaya-Putra, “Matrix Stiffness Primes Lymphatic Tube Formation Directed by Vascular Endothelial Growth Factor-C Regulate Lymphatic Tube Formation,” FASEB, 2021 March 27, 35:e21498. PMID: 33774872.
- Laura Alderfer, Eva Hall, Donny Hanjaya-Putra, “Harnessing Lymphatic Tissue Engineering to Modulate the Immune System,” Acta Biomaterialia, 2021 June 9. PMID:34118451.
- Zehao Pan, Loan Bui, Vivek Yadav, Fei Fan, Hsueh-Chia Chang, Donny Hanjaya-Putra, “Conformal Single Cell Hydrogel Coating with Electrically Induced Tip Streaming at an AC Cone,” Biomaterials Science, 2021, 9, 3284-3292. PMID: 33949367.
Project: Core-shell nanoparticles for targeted therapy against metastatic breast cancer cells in vitro and in vivo
Faculty mentors:
Professor Prakash D. Nallathamby • Aerospace and Mechanical Engineering • B02D McCourtney Hall • 631-7868 • Prakash.D.Nallathamby.1@nd.edu (Send applications to Professor Nallathamby)
Professor Paul Helquist • Chemistry and Biochemistry • 361 Stepan Hall • 631-7822 • phelquis@nd.edu
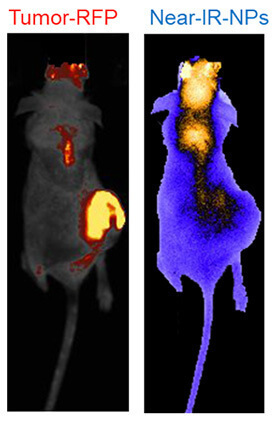
We intend to target non-targetable cancer cells by using label-free magnetic nanomaterials at the cell membrane to selectively permeabilize the more compliant cancer cell membranes but not the stiff healthy cell membranes.
Systemic delivery of chemotherapeutics is toxic to all cells but more so to cancer cells due to the high metabolic rate of cancer cells. But with the recent advances in biophysics, it is well documented that cancer cells have significantly different cell membrane stiffness in comparison to healthy cells. Cancer cell membranes are less stiff, more compliant and the cells as a whole are easily deformable. The difference in membrane physical properties has been exploited by us to selectively kill triple negative breast cancer cells, ovarian cancer cells, prostate cancer cells, and esophageal cancer cells, while avoiding toxicity to normal cells.
While the cancer cell permeabilization and cancer cell-specific drug delivery have been validated in vitro, it is not known how effective our technology will be in vivo. Therefore, in this proposed study we will validate a label-free magnetic nanocarrier system that will localize to cancer cells without the need for prior knowledge of the tumor location in vivo. We are exploiting this unique cancer cells targeting property of our nanomaterials for selective drug-delivery, gene delivery, and CAR-T targeting of cancer cells.
In this project the student will get exposure to (a) synthesis of novel nanoparticles, (b) sterile cell culture techniques, (c) execution of multiple modes of drug delivery from the nanoparticles (ON-Demand, stimuli dependent, enzymatic release, etc.) and (d) execution of in vitro cell and in vivo mouse studies that show selective targeting of therapeutic nanoparticles to metastatic breast cancer cell and improved survivability in the sample group. This project is open to applicants with a science or engineering background.
Suggested readings:
- Waters, M.; Hopf, J.; Tam,E.; Wallace, S.; Chang, J.; Bennett, Z.; Aquino, H.; Roeder, R.K.; Helquist,P.; Stack, M.S.; Nallathamby, P.D. “Biocompatible, Multi-Mode, Fluorescent, T2 MRI Contrast Magnetoelectric-Silica Nanoparticles (MagSiNs), for On-Demand Doxorubicin Delivery to Metastatic Cancer Cells.” Pharmaceuticals 15, 1216 (2022)
- Nallathamby, P. D.; Hopf, J.; Irimata, L. E.; McGinnity, T. L.; Roeder, R. K., Preparation of fluorescent Au-SiO2 core-shell nanoparticles and nanorods with tunable silica shell thickness and surface modification for immunotargeting. Journal of Materials Chemistry B 2016, 4 (32), 5418-5428.
Project: Phage-mimicking nanoparticles as broad spectrum antibacterials
Faculty mentor:
Professor Prakash D. Nallathamby • Aerospace and Mechanical Engineering • B02D McCourtney Hall • 574-631-7868 • Prakash.D.Nallathamby.1@nd.edu
In collabotation with W.M. Kick Center for Transgene Research.
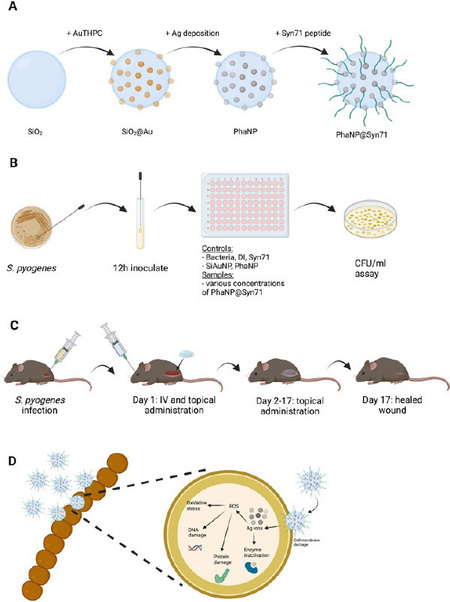
The development of antibiotic resistance and the resulting emergence of multidrug-resistant bacteria has become one of the main threats in the public health system, commonly leading to nosocomial infections. Many researchers have turned their focus to developing alternative classes of antibacterial systems based on various nanomaterials. We have developed an antibiotic-free nanoparticle system, inspired by naturally occurring bacteriophages, to fight antibiotic-resistant bacteria. Our phage-mimicking nanoparticles (PhaNPs) display structural mimicry of protein-turret distribution on the head structure of bacteriophages. By mimicking phages, we are able to take advantage of their evolutionary constant shape and their high antibacterial activity while avoiding immune reactions of the human body, potentially caused by phages. We synthesized modularly assembles core-shell nanoparticles, with a silica core conjugated with silver-coated gold nanospheres and capped with antibacterial peptides or antibacterial polymers. We will test the different nanoparticle variants against ESKAPEE class of pathogens.
In this project the student will get exposure to (a) synthesis of novel nanoparticles, (b) sterile cell culture techniques, (c) execution of multiple models of nanoparticle formulations for antibacterial testing (ON-Demand, stimuli dependent, topical, intravenous, etc.) and (d) execution of in vitro cell and in vivo mouse studies that show low dose, high-efficacy antibacterial activity and improved biocompatibility in the sample group. This project is open to applicants with a science or engineering background.
Suggested readings:
- Peptide-conjugated phage-mimicking nanoparticles exhibit potent antibacterial action against Streptococcus pyogenes in murine wound infection models. ChemRxiv. Cambridge: Cambridge Open Engage; 2023; (2023) In final review at Nanoscale Advances Olesk J, Donahue D, Ross J, Sheehan C, Bennett Z, Armknecht K, Kudary C, Hopf J, Ploplis V.A, Castellino F.J, Lee S.W, Nallathamby, P.D. ChemRXiv
- Phage-mimicking antibacterial core–shell nanoparticles. Nanoscale Advances, (2019), 1, 4812–4826. J. Hopf, M. Waters, V. Kalwajtys, K. E. Carothers, R. K. Roeder, J. D. Shrout, S. W. Lee and P. D. Nallathamby
Project: Squeezing light energy into small volumes with gold and silver nanoparticles
Faculty mentor:
Professor Svetlana Neretina • Aerospace and Mechanical Engineering, Chemistry and Biochemistry • 370 Fitzpatrick Hall • 574-631-6127 • sneretina@nd.edu
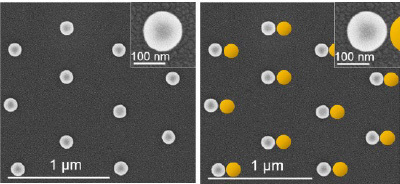
Gold and silver nanoparticles, when illuminated, have the remarkable property of being able to “squeeze” light energy into a volume that is much smaller than the wavelength of light. This ability is being widely exploited in emerging applications in areas where these nanoparticles act as (i) sensors for cancer, cardiovascular disease, explosives, and hazardous pollutants, (ii) agents that enhance solar cell efficiencies, and (iii) photocatalysts that increase the rate of chemical reactions that are performed under illumination. Fully exploiting this capability requires that such nanoparticles be placed in very close proximity to each other without actually touching. With the distances required between nanoparticles being less than five nanometers, the formation of these so-called nanogaps is quite challenging. Our research team has developed a straightforward benchtop technique for producing periodic arrays of spherical gold and silver nanoparticles such as the one shown in the image on the left. The goal of this summer’s NURF project is to develop the techniques needed to place a second nanoparticle to the right of every arrayed structure as shown schematically in the image on the right. This so-called “dimer” array is a far more valuable product because of the nanogaps formed between particles. A NURF fellowship recipient will team up with a graduate student to forward this project where his/her primary responsibilities will be nanofabricating nanoparticle arrays and synthesizing the nanoparticles used in the dimer assembly process. Applicants should have a strong background in chemistry or materials science with excellent hands-on skills.
Project: Fabrication of HfO2 ferroelectric capacitor with low coercive field
Faculty mentor:
Professor Kai Ni • Electrical Engineering • 272 Fitzpatrick Hall • 574-631-8411 • kni@nd.edu
HfO2-based ferroelectric memory rises as a prime candidate for nonvolatile memory due to its superior energy efficiency and scalability. However, one challenge with it is the relatively high coercive field (usually around 1.2MV/cm), which hinders its application as a CMOS logic compatible memory and thus requires complex write circuitry. Therefore, there is growing interest in reducing the coercive field of HfO2 to around 0.5MV/cm. Recent reports show some promising directions. In this project, we will explore the different experimental approaches to reduce the coercive field and evaluate the performance of demonstrated ferroelectric capacitors. The student will work closely with ND graduate students on literature review, device fabrication, and measurement. Applicants with knowledge of semiconductor physics and nanofabrication are preferred.
Project: Developing testing printed circuit board (PCB) for FeFET memory array
Faculty mentor:
Professor Kai Ni • Electrical Engineering • 272 Fitzpatrick Hall • 574-631-8411 • kni@nd.edu
The HfO2-based ferroelectric field effect transistor is a promising nonvolatile memory technology that offers energy efficiency and high performance. To get realistic statistics of the memory technology, it is necessary to evaluate the overall performance of the whole array. In this project, the student will be expected to develop peripheral circuitry on a PCB to support testing of a developed FeFET array, including row and column decoder, write driver, and sense amplifiers. With such an infrastructure, the student will study the programming of FeFET, especially evaluating the write-and-verify schemes, and implementing matrix-vector-multiplication (MVM) in analog domain for neural network acceleration. The student also will work on literature review, PCB design, and measurement. Applicants in electrical engineering with knowledge of PCB design and testing are preferred.
Project: Adiabatic reversible logic and clocking systems for ultra-low power electronics
Faculty mentors:
Professor Gregory Snider • Electrical Engineering • 275 Fitzpatrick Hall • 574-631-4148 • gsnider@nd.edu
Professor Alexei Orlov • Electrical Engineering • 227 Stinson-Remick Hall • 574-631-8079 • aorlov@nd.edu
Anyone who owns a laptop knows that power dissipation and the associated heat are a problem for the microelectronics industry. As electronic devices scale down in size, they use less power (and hence energy), but is there a lower limit to the energy that must be dissipated by each device? This project is investigating adiabatic reversible logic as a way to recycle the energy used in computation rather that dissipate it as heat. The project will also involve MEMS clock generators that will be able to supply and recycle the energy used in computational devices. Together the logic and clocking can reduce power dissipation far below that possible with conventional approaches.
The student working on the project will be testing a microprocessor circuit that was fabricated at a commercial foundry, and making measurements of power dissipation. In addition, the student will work in the cleanroom on fabrication of resonator devices, and on measurements of completed devices. Applicants studying electrical engineering, physics, or computer science students are preferred. Some knowledge of programming and soldering is helpful.
Project: Heterostructure assembly, nanofabrication and characterization of low-dimensional materials
Faculty mentor:
Professor Petr Stepanov • Physics and Astronomy • 436 Nieuwland Science Hall • 574-631-9454 • pstepano@nd.edu
Within the scope of this project, the student will perform thin layer isolation and nanoassembly of the two-dimensional heterostructures followed by the cleanroom fabrication. The purpose of the project will be to improve the nanofabrication workflow for air-sensitive materials. This will include 2D superconductors, semimetals, semiconductors, or magnets. The student’s work will comprise the basis for the spectroscopic studies of these materials in the far- and near-field optical cryostats.
We are searching for a highly motivated candidate with strong undergraduate knowledge in physics and/or engineering disciplines who is willing to learn about low-dimensional materials. During the project, the successful candidate will acquire necessary skills widely applied across the fields in condensed matter physics.
Examples of work
- Stepanov, P. et al. Competing Zero-Field Chern Insulators in Superconducting Twisted Bilayer Graphene. Phys. Rev. Lett. 127, 197701 (2021).
- Stepanov, P. et al. Untying the insulating and superconducting orders in magic-angle graphene. Nature 583, 375–378 (2020).
- Hesp, N. C. H. et al. Observation of interband collective excitations in twisted bilayer graphene. Nat. Phys. 17, 1162–1168 (2021).
Projects:
Evolution of strongly interacting populations
Failure and death of complex systems
Faculty mentor:
Professor Dervis Vural • Physics and Astronomy • 384G Nieuwland Science Hall • 574-631-6977 • dvural@nd.edu
We are a theoretical group that works on the interface between statistical mechanics and biology. Currently the focus of our group is in two categories of problems:
- First, evolution of strongly interacting populations, particularly when stochastic factors are as influential as selection events. For example, we would like to understand how ecological interactions form and evolve, or what role phenotypic diversity plays.
- Secondly, we are interested in the failure or death of complex systems: We study how complex networks respond to the malfunction of one or few crucial components, and how these malfunctions spread.
For more details, see https://www3.nd.edu/~